Synthesis of modified nucleoside triphosphates and polymerase construction of functionalized nucleic acids
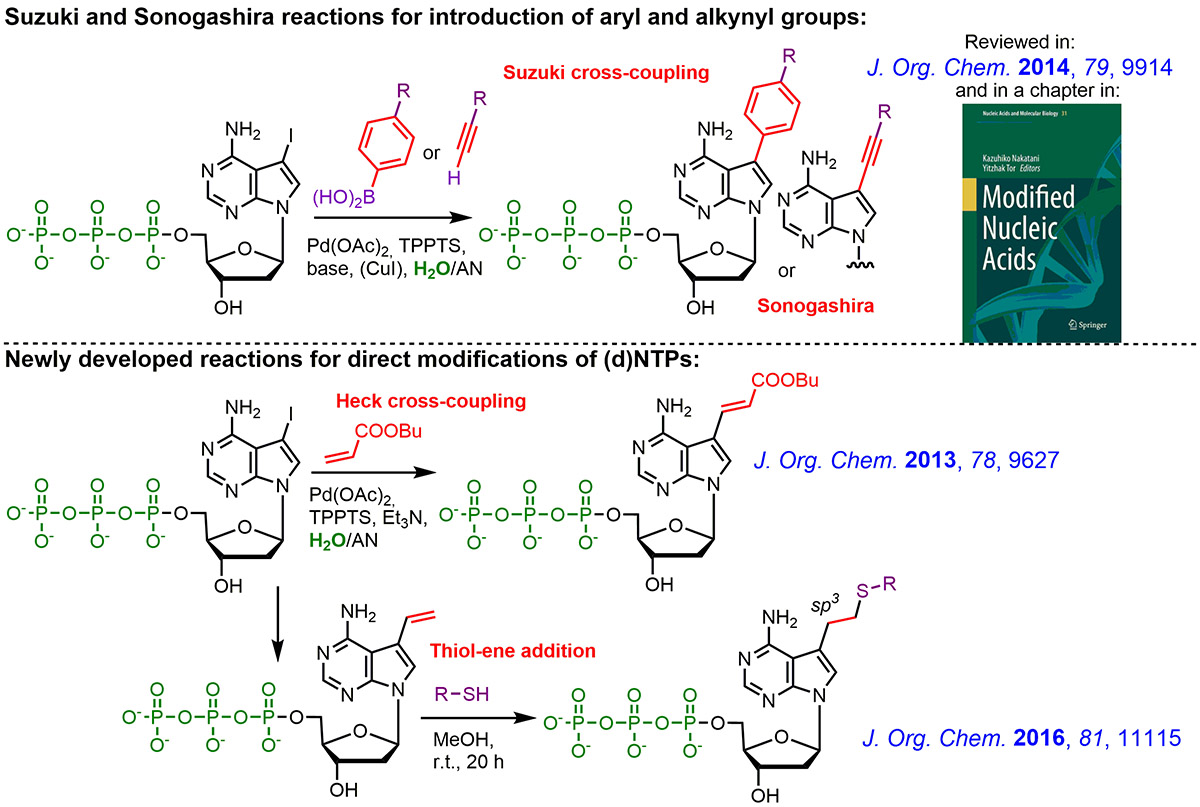
Novel efficient and expeditious two-step approach for the construction of functionalized nucleic acids was developed in our group. It is based on direct modification of nucleoside triphosphates followed by enzymatic incorporation of the modified dNTPs by DNA polymerase. We developed several types of aqueous-phase cross-coupling reactions of halogenated (d)NTPs or other reactions (click reactions, thiol-ene etc.) for attachment of selected modifications, labels or functional groups.
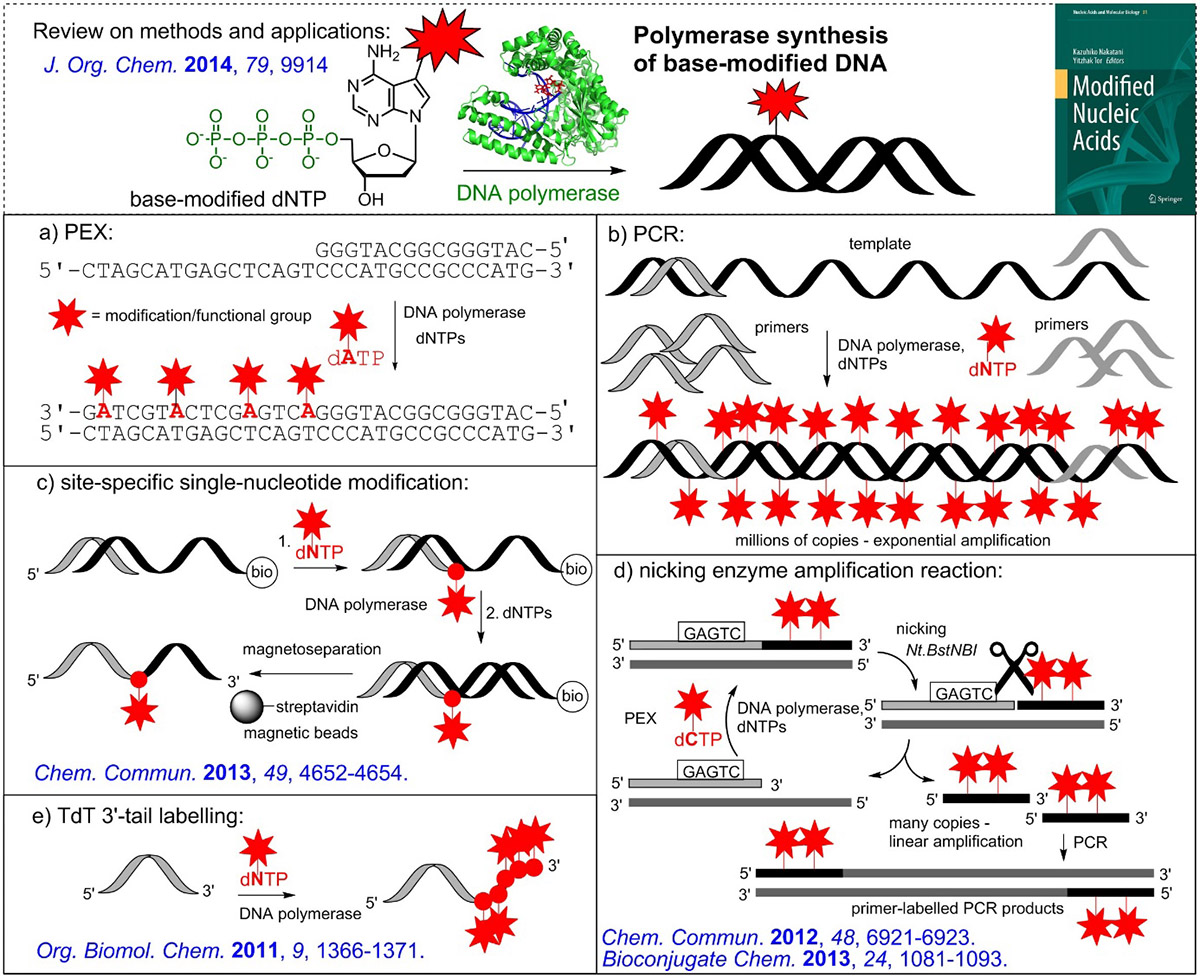
Modified (d)NTPs are then used in polymerase synthesis of modified nucleic acids. Primer extension reactions are used for the incorporation of the just one or several modified nucleotides in specific positions and, in combination with magnetoseparation of biotinylated template, to isolate single stranded ONs. PCR can be used to get larger DNA duplexes with a high degree of modifications. Terminal transferase is used for tail-labeling of 3'-end of ON, whereas Nicking Enzyme Amplification Reaction is used for synthesis of short single-stranded ONs. Recently, also synthesis of base-modified RNA was developed using T7 RNA polymerase and base-modified ribonucleoside triphosphates.
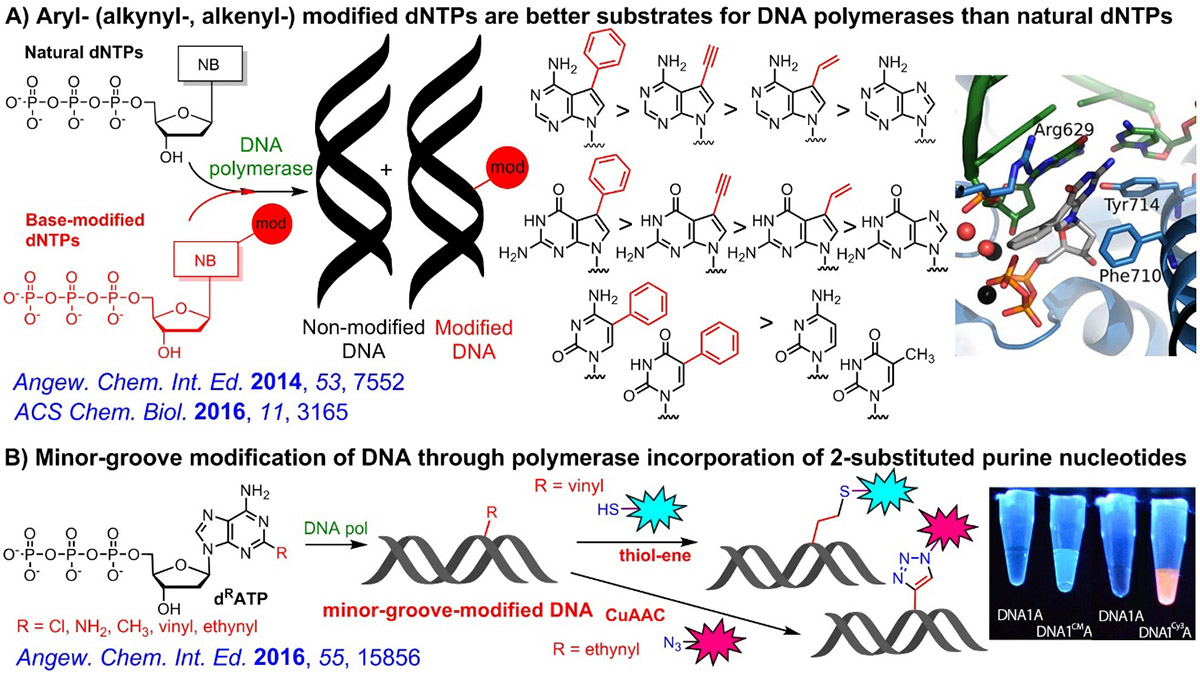
Through systematic study, we identified some base-modified dNTPs (in particular 7-aryl-7-deazapurine dNTPs) which are better substrates for DNA polymerases compared to natural non-modified dNTPs because of their higher affinity to the active site of the polymerase. Recently, we also developed enzymatic synthesis of DNA modified in the minor groove.